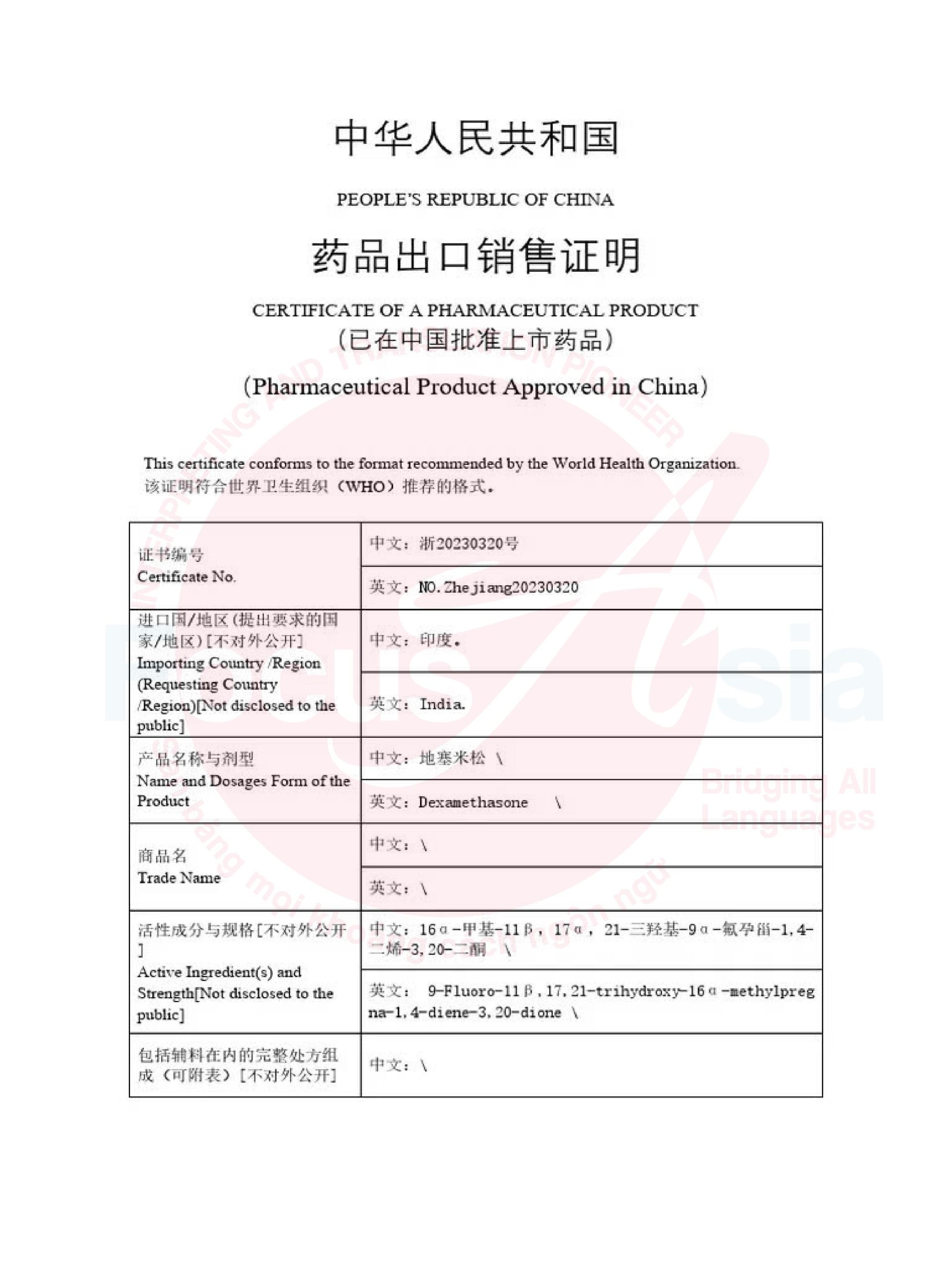
1. Why is translation and notarization of CPP essential?
The Certificate of Pharmaceutical Product (CPP) is a critical document for:
-
International export and distribution: Meeting legal requirements in importing countries.
-
Pharmaceutical product registration: Facilitating application submissions to foreign health authorities.
-
Product quality assurance: Demonstrating that the product meets quality standards and has legal market authorization.
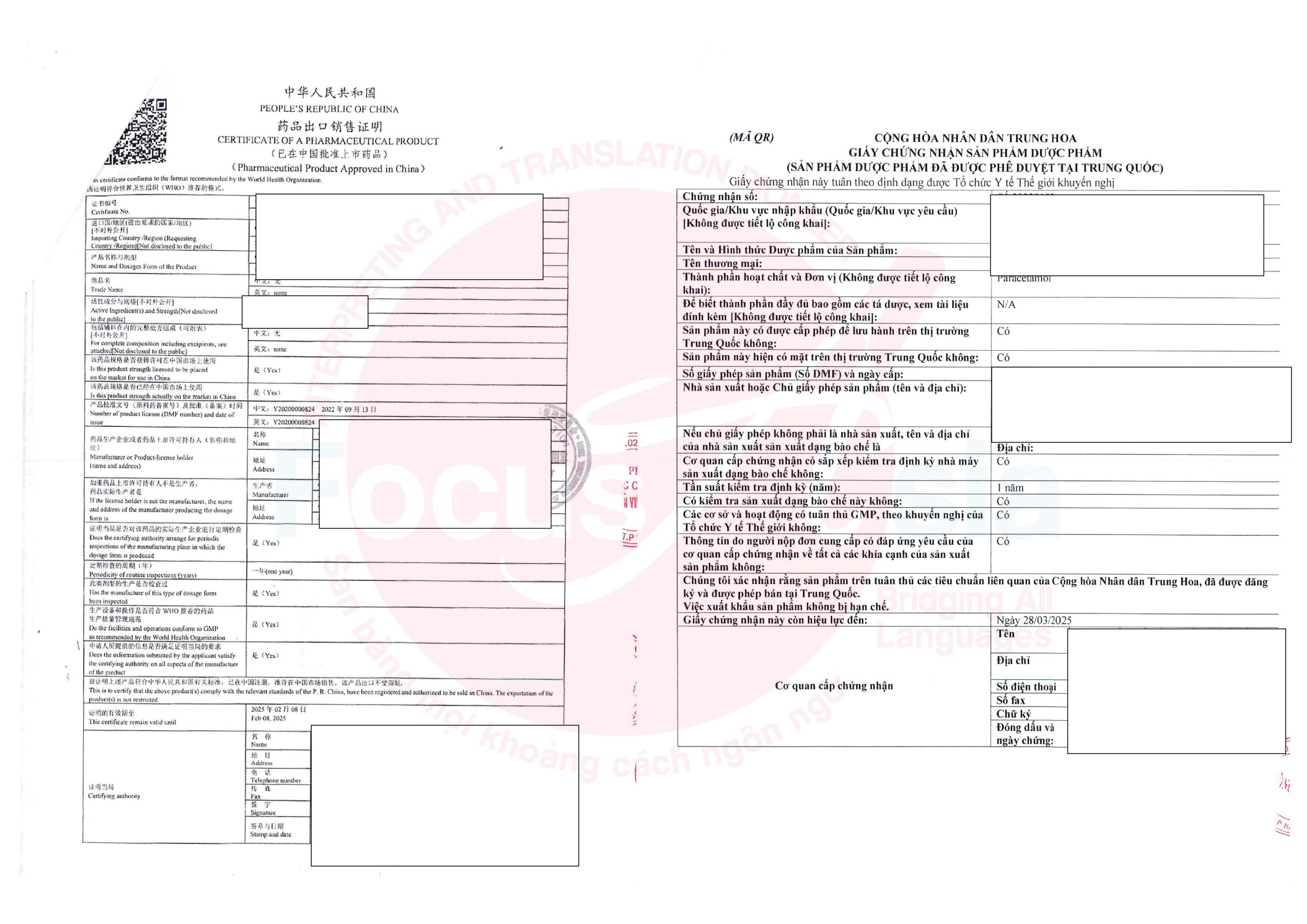
2. Translation and notarization process at Focus Asia Translation and Interpretation Company
Step 1: Document submission
-
Receive the original or a copy of the CPP from the client.
-
Define the target language and notarization requirements.
Step 2: Professional translation
-
Experienced translators specializing in medical and pharmaceutical terminology ensure precise translations.
-
Perform thorough quality checks to maintain technical accuracy.
Step 3: Document notarization
-
Collaborate with notarization authorities to validate the document legally.
Step 4: Delivery of documents
-
Provide the translated and notarized document to the client on time.
3. Why choose Focus Asia Translation and Interpretation Company?
-
Pharmaceutical expertise: Extensive experience in translating medical and pharmaceutical documents.
-
Legal assurance: Accurate translations and legally certified notarization.
-
Time efficiency: Fast processing times to save clients’ valuable time.
-
Data security: Commitment to maintaining the confidentiality of client information.
Contact Us Today
Trust Focus Asia Translation and Interpretation Company for professional and reliable translation and notarization services for your Certificate of Pharmaceutical Product.


















